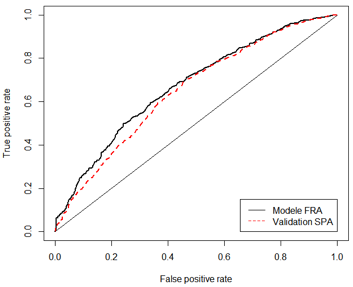
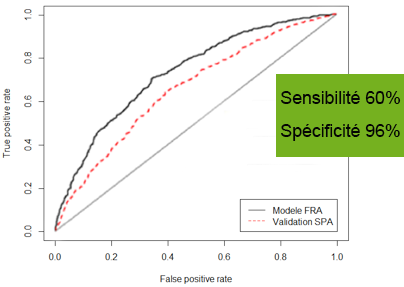
The risk of developing cutaneous melanoma is associated with clinical risk factors (skin and eye colour, phototype, number of moles etc.) and genetic factors (predisposition genes or protective genes against disease).
MELAPRED is a test that simultaneously integrates both genetic factors and clinical risk factors, which thanks to a specific algorithm enables the calculation of a melanoma risk score de mélanome (between 0 and 100) for the patient.